Initial Study of Commercial Innovation in the Lower Limb O&P Industry
JunJay Tan, BS, and Dava J. Newman, PhD
Technology and Policy Program, Man Vehicle Laboratory—Massachusetts Institute of Technology
Cambridge, MA
ABSTRACT
To gain insight into policies that would spur commercial innovation in lower limb prostheses/orthoses, this exploratory study analyzes the development process and times of 14 products representing technology innovations in the market since the 1980s. It concludes that research collaborations with large firms, industry-insurance organization collaborations, and increased federal funding for orthosis research may be significant for advancing innovation.
KEYWORDS
Innovation, Research and Development, Product Development
INTRODUCTION
Understanding the dynamics of innovation in lower limb orthoses and prostheses (O&P) is important for several reasons. First, growing numbers of aging and war veteran populations will require O&P. This has led to increased public interest and investment in O&P research. Yet questions remain about how best to promote innovation that leads to affordable commercial products. Second, the O&P professional and market structure is different from that of other medical devices, which might necessitate different public policies to spur innovation. This exploratory study aims to identify further directions in lower limb O&P innovation research.
We focus specifically on lower-limb O&P because these devices hold the potential to address mobility impairments in disabled patients and amputees, which in turn would allow great functional and health benefits. For example, living a wheelchair-bound life can lead to medical problems such as osteoporosis, cardiovascular deconditioning, and overuse injuries [1, 2], while psychologically, the ability to walk and stand at eye-level can increase self-esteem and confidence [3].
DEFINITIONS AND SCOPE
The scope of this paper is lower limb orthoses and prostheses (hereafter referred to as O&P) that are commercially available in the U.S. By lower limb, we refer specifically to devices that replace or address functions of the knee, ankle, and hip. By O&P, we refer to products that interface externally to the body (e.g., ankle-foot orthoses, leg prostheses). We focus only on commercially available products. Since we are concerned with basic mobility, we also only focus on products that address chronic mobility concerns, such as paralysis and limb loss, and not sport performance or rehabilitation. Lower limb prostheses replace the function of a missing body part. In contrast, orthoses support or augment the function of an existing body part [4]. Such devices include ankle-foot orthoses (AFO) and knee-ankle-foot orthoses (KAFO). Outside our scope are foot orthotics (shoe inserts).
Innovation may be classified into product innovation, process innovation, and organizational innovation. Here, we focus on product innovation—the development and commercialization of new products or services fulfilling unmet customer needs.
BACKGROUND
Research into the assistive technology industry is sparse. The U.S. Dept. of Commerce performed a survey-based study of the U.S. assistive technology industry and concluded that the industry was hampered by “cumbersome public and private insurance program participation procedures,” “outdated compensation methodologies,” and high fragmentation of small companies [5]. The last decade has seen increased consolidation in the O&P industry as well [6, 7].
O&P is a small part of the medical device market. Otto Bock estimated that 2004 revenue among all U.S. prosthetics manufacturers was around $300 million annually [8], while revenue of the total U.S. medical device market is estimated at around $57.6 billion [9].
Several factors differentiate O&P from other medical device markets. One difference is the educational infrastructure for orthotists and prosthetists (O/P’s). O/P’s work hands-on with patients to fit and sometimes build O&P, yet only eight universities have NCOPE accredited U.S. O&P bachelors/postgraduate programs (though one can become a certified O/P without graduating from such programs). Further, few O/P’s pursue PhD’s, meaning O&P experts are generally from outside the profession [10]. One survey study found that a large percentage of certified O/P’s do not conduct research. Those that do generally spend less than 20% of their time on research [11]. This leads to disconnect between academic researchers and clinicians. For example, in a Keck Conference orthoses task group comprised of 12 industry leaders and engineering and medicine academics, about half the group had no experience with people who might use orthoses daily [12].
The O&P field also lacks a firm scientific knowledge base [11]. This makes outcomes harder to define, meaning expensive reimbursements are hard to justify. This is significant for expensive next generation O&P products. The lack of firm scientific grounding means that more clinical experimentation is required to develop innovative O&P products.
Patients purchasing O&P are reimbursed through private insurance or Medicaid/Medicare [13]. In the U.S., O&P are covered under durable medical equipment (DME) rules as defined in Title XVIII of the Social Security Act. After they pass certain requirements, they are assigned L-Codes under Medicare/Medicaid, which determines reimbursement rates.
Although recent press highlights interest in O&P research and development (R&D), the actual funding situation is unclear. For example, one report stated that although Veterans Administration R&D budget increased from 2001-2004, it fell in 2005 and has remained flat ever since [14]. The government invested heavily in prosthetics R&D after World War II [15] but then withdrew from actively shaping prosthetic R&D, leaving it to manufacturers and prosthetists [16], though federal funding for academic projects still exists.
A recent spurt of innovation in prostheses has brought about many new technologies, while a similar scenario has not occurred for orthoses. Scientific and technological issues are one reason for this difference. Orthoses cover a variety of physical and neurological disabilities while prostheses do not. Griffin also argues that it is harder to design a device that works in conjunction with an existing body part than a device that replaces a body part [17]. A local orthotist also mentioned that reimbursement rates for orthoses are generally lower than that for prostheses [18]. But other social/market factors may be important as well, which we hope to gain some insights into.
HYPOTHESES
Because of weak scientific grounding for O&P, we hypothesized that either (1) independent inventors with direct knowledge of the disability who could easily test devices on family members or themselves, or (2) university-to-industry licensing agreements would yield the most innovative products, since industries are unlikely to take big internal development risks in a small market. Other questions we sought to gain insight to through our study included
- Does consolidation in industry lead to more internally-driven innovations?
- Does the innovation process differ between orthoses and prostheses?
- What types of collaborations seem to produce innovations?
METHODOLOGY
We aimed to identify representative technologies in the market that have appeared since the 1980s. We attempt to trace each technology to its origin, or, if this product was not widely used, to its widely used representative product. The first version of each product is examined. If several products exhibiting the same technological innovation arrived on market at approximately the same time, we profiled multiple products since they are probably not derivative products. Significant technologies and products were identified by reviewing trade journals and magazines, company catalogs (see Table 1), and conversations with local orthotists and prosthetists [18, 19].
| Journals | Company Catalogs |
|
|---|---|---|
| Journal of Prosthetics and Orthotics | College Park Industries | Hangar Orthopedic Grp. |
| Prosthetics and Orthotics International | Endolite/Exolite/Chas. A Blatchford & Sons | Orthomerica |
| Journal of Rehabilitation Research and Development | Ossur | Allard USA/Camp Scandinavia |
| Kingsley Mfg Co. | Ultraflex | |
| Ohio Willow Wood | Orthofix | |
| SPS | Becker | |
| Otto Bock | Townsend Design | |
| Trade Magazines | Freedom Innovation | Euro International |
| The O&P Edge | Fillauer Companies | Boston Brace |
| inMotion (Amputee Coalition of America) | Euro International | Daw Industries |
| O&P Almanac | Bledsoe Brace Systems | Seattle Systems |
| DonJoy | Medi | |
DATA AND RESULTS
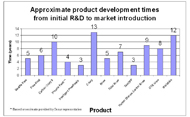
We obtained the development/commercialization environment and pathway, market introduction date, approximate development time to commercialization (where available), and significance/awards won by each product profiled through searching literature and contacting companies. Technological developments within our time frame of consideration and the products representing those developments are as follows. For prosthetic feet, these include new materials that allow energy storage (Seattle Foot [20-23], Flex-Foot [23-25], Carbon Copy II [26, 27]) and microprocessor control (Proprio Foot [28-30]). For prosthetic knees, these include various mechanical stance control designs (Total Knee [31, 32]), microprocessor control (Intelligent Prosthesis [33-36], C-leg [34, 37]), microprocessor control of “smart fluids” (Rheo [38]), and power augmentation (Power Knee [39, 40]). For orthoses, these include dynamic response AFOs (ToeOFF [41, 42]), stance control knee orthoses (UTX, Horton Stance Control Knee Joint, Fillauer Swing Phase Lock, microprocessor stance control knees (E-knee), and functional electrical stimulation (WalkAide). A total of 14 products were analyzed, eight prostheses and six orthoses. Summaries of our data are shown in Tables 2 and 3, and development times are shown in Figure 1.
| Prosthetic and introduction date | Development | Description | Awards/ Significance |
|---|---|---|---|
Seattle Foot (1985) |
Created by an orthopedic surgeon/faculty member at the University of Washington engineers at Boeing, and a private design firm with funding from the VA. Commercialized through an existing small firm (Model & Instrument Works). |
Energy-storing foot made of a Delrin keel, a Kevlar reinforcement pad, and an exterior polyurethane foam matrix; |
First energy-storing foot |
Flex-Foot (1987) |
Developed in the U.S. by a trans-tibial single amputee with engineering/ prosthetics background in collaboration with an engineer. Amputee developed product on own time. Testing largely done on amputee himself. Inventor and his partners created a startup to commercialize the product. |
Energy storing foot made of carbon-fiber composite designed in a unique C-shape |
Widely used by world-class Para-Olympic athletes |
Carbon Copy II (1984) |
Internally at Ohio Willow Wood (U.S.) |
Energy storing foot made of two carbon fiber deflection plates. |
Various trade journal awards, high patient acceptance |
Proprio Foot (2006) |
Ossur (Iceland) in collaboration with DesignEdge. Sold by Ossur. |
Microprocessor controlled energy storing foot. |
Popular Science Best of What’s New; da Vinci Award |
Total Knee (1993) |
Developed in Sweden by an independent inventor with engineering background who tested the devices on an amputee family member, then commercialized the knee through a startup company |
Polycentric design, weight activated stance control based on geometric design, hydraulics control knee resistance |
R&D Magazine top 100 products; |
Intelligent Prosthesis (1993) |
Developed internally by Chas A. Blatchford & Sons (UK) in collaboration with users and UK’s National Health Service (NHS) |
Microprocessor-controlled knee prosthesis adjusts knee resistance to adapt to various walking speeds through a pneumatic system. |
First microprocessor-controlled knee prosthesis, 1996 Prince of Wales Award for Innovation, Queens Award for Technological Achievement |
C-leg (1999) |
Initial concept and hardware development by inventor at a university (Canada), then licensed to Otto Bock (Germany) for refinement and commercialization |
Microprocessor-controlled knee prosthesis adjusts knee resistance to adapt to various walking conditions through a hydraulic system. |
da Vinci Award |
Rheo (2005) |
Developed by university lab in collaboration with Ossur, then refined and commercialized by Ossur |
Microprocessor-controlled magnetorheological fluid knee prosthesis adjusts knee resistance to adapt to various walking conditions |
Frost & Sullivan Technology of the Year Award, Central European Award |
Power Knee (2006) |
Developed by small firm Victhom Human Bionics (Canada), which then partnered with Ossur to market the product. |
Microprocessor-controlled motorized knee can add power to human gait. |
First commercially available knee that adds power during gait |
| Orthosis and introduction date | Development | Description | Awards/Significance |
|---|---|---|---|
Allard ToeOFF Dynamic AFO (1997) |
Developed by university researchers in collaboration with Camp Scandinavia, a large orthopedic company. |
Dynamic response AFO designed for drop foot. Made of carbon fiber, Kevlar, and glass fiber, which stores energy at heel strike and returns some of it during toe-off. |
First dynamic response AFO |
Horton Stance Control Orthotic Knee (2002) |
Initial development by NASA engineers, then further developments were done in collaboration with a small, private orthotics company (Horton) through NASA’s technology transfer program. Licensed to the small company, which now commercializes it.
|
Weight-activated mechanical stance control knee joint using NASA technology. Locks when weight is on the heel, but allows the knee to bend otherwise. For patients with loss of muscle control due to stroke or accident. |
Stance control knee orthosis |
Fillauer Swing Phase Lock knee (2002) |
Developed privately by Basko Healthcare, a small firm in the Netherlands. |
Angle-activated mechanical stance control knee joint. It locks/unlocks depending on the joint angle. |
Stance control knee orthosis |
Becker E-Knee (2002) |
Developed by Becker in consultatation with a private practice clinic |
Microprocessor controlled, weight-activated stance control orthosis. Can lock at any angle during gait. |
First microprocessor-controlled stance control knee orthosis |
Becker UTX knee (1995) |
Initially developed at university as part of theses, then sold by Ambroise Holland, a private firm in Sweden in 1995. Distributed in U.S. by Becker. |
Mechanical stance control orthosis. At the end of swing phase, as knee reaches full extension, a ratchet locks the knee. Cable linkages then unlock the knee at the end of stance phase. |
First “reliable” commercially available stance orthosis |
WalkAide (FES) (2006) |
Developed by a professor at the University of Alberta. After several failed attempts to commercialize the product through a startup, the product was licensed to Hangar Orthopedic. |
Uses an accelerometer to sense tilt in the disabled foot. During the programming stage, the clinician programs the device to apply proper functional electrical stimulation to the peroneal nerve based on accelerometer readings. Device is designed to treat drop foot. |
Da Vinci Award, U.S. Orthopedics Product of the Year Award from Frost & Sullivan |
| Category | Product | Development | Commercialization |
|---|---|---|---|
Prosthesis |
Seattle Foot |
University/nonprofit lab, large firm, small firm collaboration |
Small firm |
|
Flex-Foot |
Independent inventor |
Startup |
|
Carbon Copy II |
Large firm |
Large firm |
|
Proprio foot |
Large firm |
Large firm |
|
Intelligent Prosthesis |
Large firm |
Large firm |
|
C-leg |
University |
Large firm |
|
Rheo |
University, Large firm collaboration |
Large firm |
|
Power Knee |
Small firm |
Large firm |
|
Total Knee |
Independent inventor |
Startup |
Orthoses |
ToeOFF |
University, Large Firm |
Large firm |
|
Horton Stance Control Orthosis |
Government, Small firm |
Small firm |
|
Swing Phase Lock |
Large firm |
Large firm |
|
E-knee |
Large firm |
Large firm |
|
UTX knee |
University |
Small firm |
|
WalkAide |
University |
Large firm |
DISCUSSION AND CONCLUSIONS
We note that our data only presents trends in the O&P market, since development times are approximate and we did not perform detailed case studies.
It is interesting that for prostheses, many products were developed internally by large firms or by independent inventors, whereas we did not hypothesize a strong link between large firms and innovation. Even for two of the three university-developed prostheses (Seattle Foot and Rheo), large firms still collaborated with academic labs. We expected most developments to have originated solely in academia (rather than through academic-industry collaborations), then licensed to a large firm with the ability to refine and market the product. It is also interesting that one of the significant developments in knee prostheses—the use of microprocessors—was developed internally by a large commercial firm (the Intelligent Prosthesis (IP) by Blatchford). This may be because Blatchford owns clinics and product development centers, which allows it to leverage both clinical expertise and manufacturing expertise. Technology-related academic labs may lack clinical knowledge, while clinical labs may lack technology and manufacturing knowledge. Blatchford also worked closely with UK’s NHS regarding reimbursement, which helped reduce market uncertainty. The IP also took about three years to go from development to commercial product, while the C-leg, developed initially by an inventor working in academia, took over a decade, and the Rheo, developed through a university lab/large firm collaboration, took about five years. This suggests that increasing system complexity of electromechanical O&P systems—requiring knowledge in software, mechanical, and electrical engineering—necessitates close collaboration between researchers, clinicians, and industry. However, independent inventors might still make significant contributions to more affordable, “simpler” designs that larger firms and universities overlook in lieu of more “high-tech” solutions.
Our dataset for orthoses is very small, but we make some preliminary observations that suggest further research avenues. First, no orthotic device studied arose from a startup. This suggests that public policies should target orthoses development and commercialization efforts, as supported by our literature review. Second, during our literature search we found many articles about physically active war veterans who eagerly test the latest technologies (e.g., [43]). This, coupled with a large paralympic community, leads us to suggest that there may exist a large community of lead users for prostheses that is absent for orthoses. The importance of lead user communities in promoting O&P innovation is a future question.
One final observation is that energy storing orthoses (e.g., ToeOFF) arrived on the market about a decade after similar technology was introduced in prostheses (Seattle Foot). As consolidation between O&P companies continues, there might be more technology transfer between prostheses and orthoses development, allowing more innovations in orthoses. One example is Victhom attempting to adapt Power Knee technology to a powered orthosis.
ACKNOWLEDGEMENTS
The authors thank representatives at NOPCO in Boston for their assistance, as well as the Portuguese government and the MIT-Portugal Program for financial support.
AUTHOR CONTACT INFORMATION
JunJay Tan, BS, Man Vehicle Laboratory, Massachusetts Institute of Technology, 77 Massachusetts Avenue, 37-219, Cambridge, MA 02139. Office Phone: (617) 258-7552. jjtan@mit.edu
REFERENCES
- Kunkel, C., et al., Effect of “standing” on spasticity, contracture, and osteoporosis in paralyzed males. Arch Phys Med Rehabil, 1993. 74(1): p. 73-78.
- van der Woude, L.H.V., S. de Groot, and T.W.J. Janssen, Manual Wheelchairs: Research and innovation in rehabilitation, sports, daily life and health. Medical Engineering & Physics, 2006. 28: p. 905-915.
- Nene, A., H. Hermens, and C. Zilvold, Paraplegic locomotion: a review. Spinal Cord, 1996. 34: p. 507-524.
- Kelly, B.M. (2007) Lower Limb Prosthetics. e-medicine.
- U.S. Department of Commerce, Technology Assessment of the U.S. Assistive Technology Industry. Feb. 2003: Washington, D.C.
- Piper Jaffray Healthcare Conference (1999) Hanger Orthopedic Group, Inc. Presents at The Piper Jaffray Healthcare Conference.
- Keegan, P., The New Bionic Man: Ossur revolutionized the business of building prosthetic legs, making amputees better, stronger, faster—and itself more profitable, in Business 2.0. Aug. 1, 2004: CNN Money.com.
- Zamiska, N., Bionic knee ‘learns’ how to walk, in Wall Street Journal. 2004, Dow Jones.
- Werner, C., Analyst reports medical device market flourishes, in Healthcare purchasing news. 2003.
- Raschke, S.U., The future of prosthetics & orthotics as a profession: the greatest threat is...?, in The O&P Edge. 2002, Western Media LLC: Northglenn, CO.
- Northwestern University PRL & RERP, Research in P&O: Are We Addressing Clinically-Relevant Problems? Report on the State-of-the-Science Meeting in Prosthetics and Orthotics. 2007, Northwestern University: Chicago, IL.
- NAP, Refine Technologies to Create Active Orthotic Devices: Task Group Description, in National Academies Keck Futures Initiative: Smart Prosthetics: Exploring Assistive Devices for the Body and Mind: Task Group Summaries. 2007, National Academies Press. p. 23-24.
- Seymour, R., Prosthetics and Orthotics: Lower limb and spinal. 2002, Baltimore: Lippincott Williams & Wilkins.
- American Association for the Advancement of Science, VA R&D Remains Flat in 2007 Budget. 2007, AAAS.
- Norton, K.M., A Brief History of Prosthetics, in inMotion. 2007, Amputee Coalition of America: Knoxville. p. 11-13.
- Pritham, C.H., Special considerations: Emerging trends in lower-limb prosthetics: Research and development, in Atlas of limb prosthetics: Surgical, prosthetic, and rehabilitation principles, American Academy of Orthopaedic Surgeons, J.H. Bowker, and J.W. Michael, Editors. 2002, American Academy of Orthopaedic Surgeons,: Rosemont.
- Griffin, J.J., Freeing the knee. Rehab Management, 2006(July).
- National Orthotics and Prosthetics Company (Longview), Personal communication. 2007: Boston, MA.
- National Orthotics and Prosthetics Company (Brookline), Personal Communication. 2007: Boston, MA.
- Illman, D.L. Pathbreakers: A century of excellence in science and technology at the University of Washington. 1996 Nov. 1996 [cited 2007 Dec. 18]; Available from: http://www.washington.edu/research/pathbreakers/1985a.html.
- Office of Development and Alumni Relations—Worcester Polytechnic Institute. 2001 Recipients. 2007 Feb. 22, 2007 [cited 2007 Dec. 12].
- Thomas, S.S., et al., Comparison of the Seattle Lite Foot and Genesis II Prosthetic Foot during walking and running. Journal of Prosthetics and Orthotics, 2000. 12(1): p. 9-14.
- Hafner, B.J., et al., Transtibial energy-storage-and-return prosthetic devices: a review of energy concepts and a proposed nomenclature. Journal of Rehabilitation Research and Development, 2002. 39(1): p. 1-11.
- Davidson, M. Artificial Parts: Van Phillips. Innovative Lives 2005 9 Mar. 2005 [cited 2007 Dec. 12]; Available from: http://invention.smithsonian.org/centerpieces/ilives/van_phillips/van_phillips.html.
- Lemelson-MIT Program. Inventor of the week archive: Flex-Foot. 2007 Jan. 2007 [cited 2007 Dec. 4]; Available from: http://web.mit.edu/invent/iow/phillips.html.
- Arbogast, R. and C.J. Arbogast, The Carbon Copy II—From Concept to Application. Journal of Prosthetics and Orthotics, 1989. 1(1): p. 32-36.
- The O&P Edge, Ohio Willow Wood Celebrates 100th Anniversary, in The O&P Edge. 2007.
- Barnett, A., iPod design looks good to medical electronics market, in electronicsweekly.com. 2007.
- New York Times. Electronic Brain in an Artificial Foot. 2006 3 Oct. 2006 [cited 2007 Dec. 5].
- Ossur Americas. Phone Conversation. 2008.
- Ossur. The love of a father—Finn Gramnas. 2006 2006 [cited 2007 Dec. 18]; Available from: http://www.oessur.de/template110.asp?PageID=111.
- Murray, C.J., Locking action makes prosthesis lighter, more reliable, in Design News. 1995, Reed Business Information.
- Royal Academy of Engineering, Design Principles: the engineer’s contribution to society. 2002, Royal Academy of Engineering: London.
- Austen, I., A leg with a mind of its own, in New York Times. 2002, New York Times Company: New York.
- Endolite. History of prosthetics. 2007 2007 [cited 2007 Dec. 21].
- Anderson, S., Design: The Millennium Collection No 10: The Blatchford Intelligent, in The Independent. 1998: London.
- Kawaleshka, D., Technical marvels may revolutionize health care: smart leg, in Maclean’s. 2002.
- Lab, M.B., Personal Communication. 2008: Cambridge, MA.
- Hoag, H., Bold strides toward bionic future, in The Gazette. 2005: Montreal.
- Perkes, C., Technology with legs, in The Orange County Register. 2006: Orange County.
- Wright & Filippis Co. Footdrop? Take a look at the latest technology. 2003 [cited 2007 Dec. 10]; Available from: http://www.firsttoserve.com/html/communicator/2003/nov_dec03/prez.html.
- Allard USA, Phone conversation. 2008.
- Mishra, R., Technology serving new war amputees, in The Boston Globe. 2005, New York Times Company: Boston, MA.